Nelsons Homeopathic Pharmacy #3
The previous instalments of this saga covered the MHRA's role in dealing with our complaints against Nelsons Homeopathic Pharmacy. Now the GPhC…
Complaints
Our complaints arose from a visit we paid to Nelsons Homeopathic Pharmacy in Mayfair, London on 12 May 2015. We were appalled by what we saw there and, in summary, we submitted the following complaints about the pharmacy, their pharmacy website and their Nelsons Natural World website, each complaint following the previous because we felt they were not dealt with properly:
Complaint 1: 28 May 2015 Joint complaint to the MHRA and GPhC about Nelsons' pharmacy and website.
Complaint 2: 28 July 2016 Second joint complaint to the MHRA and GPhC about Nelsons' pharmacy.
Complaint 3: 28 November 2016 Third joint complaint to the MHRA and GPhC about Nelsons' pharmacy.
For the background to all this, see:
Nelsons Homeopathic Pharmacy #1
Nelsons Homeopathic Pharmacy #2
Rubbing salts into the wounds of homeopathy
 So far, so good. But a major part of our complaint about Nelsons Homeopathic Pharmacy concerned the General Pharmaceutical Council (GPhC), the regulator for pharmacists and pharmacies. So far, so good. But a major part of our complaint about Nelsons Homeopathic Pharmacy concerned the General Pharmaceutical Council (GPhC), the regulator for pharmacists and pharmacies.
Our complaint was submitted to both the GPhC and the medicines regulator, the MHRA, in May 2015. We weren't sure what specific aspects each would deal with and neither, it seems, did they. The GPhC had to discuss the case with the MHRA and decide what their remit was and took legal advice.
What were we complaining about?
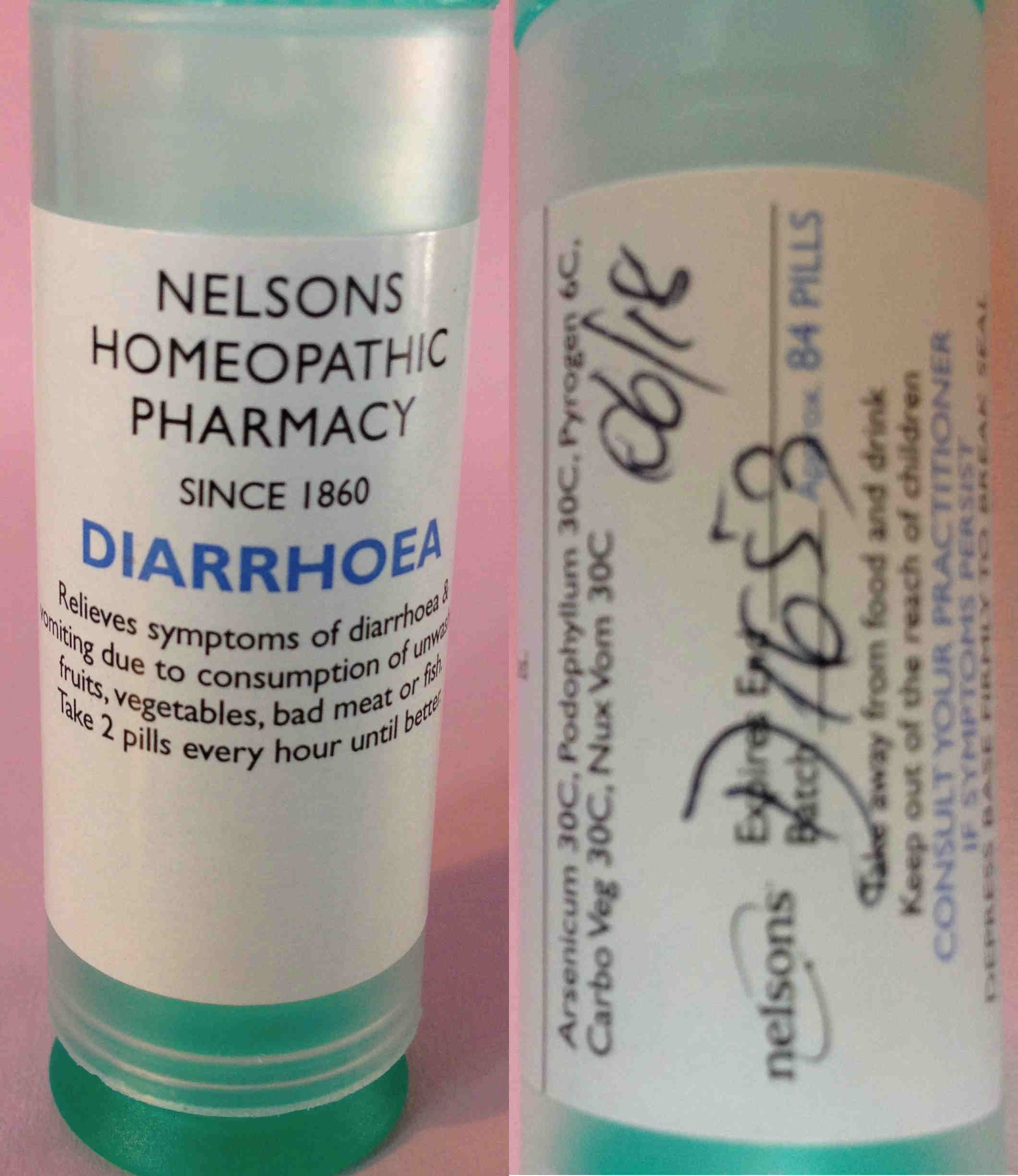
Our complaint covered a number of issues that concerned us (including the kits of homeopathic products that were on sale, tissue/cell salts, point of sale (POS) advertising, various herbal products and their price list) but we will focus on the main one here: on our first visit to Nelsons premises, Maria self-selected a tube of a homeopathic product labelled 'Diarrhoea'.
Why was this a problem?
This product is not registered and not authorised by the MHRA. As such, it is essentially an unlicensed medicine (technically, the MHRA had not determined its status but it clearly was not a registered and not an authorised homeopathic product), it should not have had indications (in this case diarrhoea), should not have been advertised, should not have been available for self-selection by a customer and should not have been sold other than in very limited circumstances — more on that later.
This tube was just one of many:

The labels on the front of the boxes tells us they contained homeopathic products for:
Aching legs
Acidity & heartburn
Alert mind
Arthritis & Rheumatism
Back ache & sciatica
Bites and stings
Bloating
Boils & carbuncles
Catarrh
Chesty cough
Cold sore relief
Computer eye strain
Concentration
Constipation
Cystitis
Detox support
Diarrhoea
Dizziness
Dry cough
Flu relief
Food poisoning
Gout relief
Grief and Bereavement
Haemorrhoids
Hangover
Hayfever & allergy
Healing combo
Healthy hair
Jet lag
Lactation blend
Mastitis
Menopause relief
Mens [sic] health
Migraine headaches
Morning sickness
Mouth ulcers
Nervous anxiety
Post flu tiredness
Rheumatism
Sports injuries
Womens [sic] health
Youthful skin
That's quite a list of medical conditions, but the one we selected was labelled:
NELSONS HOMEOPATHIC PHARMACY
SINCE 1860
DIARRHOEA
Relieves symptoms of diarrhoea & vomiting due to consumption of unwashed fruits, vegetables, bad meat or fish.
Take 2 pills every hour until better.
Arsenicum 30C, Podophyllum 30C, Pyrogen 6C, Carbo Veg 30C, Nux Vom 30C
Expires End 06/18
Batch D1653 Approx 84 PILLS
Take away from food and drink
Keep out of the reach of children
CONSULT YOUR PRACTITIONER IF SYMPTOMS PERSIST
That is a very bold therapeutic claim: as well as breaching the medicines regulations, we believed this was highly irresponsible. Note that it doesn't say to consult your GP if symptoms, just your (homeopathic) practitioner.
Ingredients
The most interesting ingredient here is the Podophyllum: also known as May Apple, Devil’s Apple, Wild Lemon and Indian podophyllum, it is a herbaceous perennial plant in the family Berberidaceae, and is poisonous. As such, it is designated as a banned and restricted herbal ingredient by the MHRA and medicines containing it are classed as Prescription Only Medicines (POM).
The label on the diarrhoea product claims it is diluted to 30C, that is, one part in 1,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000, but, of course, that depends on it having been properly manufactured. The MHRA decided it wasn't a POM because it was diluted so much.
That out of the way, the GPhC turned to dealing with our complaint.
Eventually, in February 2016, we were told the case was going to be sent to their Investigating Committee. It took a while to get our witness statements sorted out but they eventually took our statements, our full complaint, the video we had taken and the MHRA's decision to their committee. We didn't get their decision on the complaint until May 2016, one full year after we had submitted our complaint. They said:
The GPhC has reviewed all of the available information and we have concluded that the matter you have made a complaint about is not a matter that requires regulatory action. Accordingly this case file has been closed.
Considering the seriousness of the issues we complained about, we found this dismissal astonishing. However, the four reasons they gave for this decision were, we believed, entirely spurious and erroneous:
- There is no requirement for the pharmacist to identify herself, other than to display an Responsible Pharmacist notice. The video you supplied shows the Responsible Pharmacist notice behind the person serving Ms MacLachlan. In addition, the pharmacy produced the Responsible Pharmacist log to the GPhC confirming the pharmacist who was on duty that day.
- The medicines were not used inappropriately and no harm was caused.
- There is no information to suggest the sale and display of homeopathic medicine was in breach of any legislations [sic].
- The issue regarding the leaflets is a matter for the advertising unit of the MHRA, but do not breach regulations relating to the sale and supply of POMS.
The incompetence here is quite astounding. Addressing each of these points briefly (and this really didn't cover all of our complaint):
- This was irrelevant because Maria was sold an unauthorised medicinal product by someone who wasn't a pharmacist and without a prescription. We had never questioned the identity of the pharmacist.
- Goodness knows what they deemd appropriate use of the sugar pills, but we never said — and were never asked — whether we had taken any of the product or whether we had been harmed, so we had no idea how the GPhC had arrived at their conclusion.
- We provided clear video evidence of the multiple breaches — and they could easily have verified it with a site visit if they had wanted — so we are at a loss to understand this.
- None of the homeopathic products were POMs but were unauthorised medicines.
We complained again, taking apart this wholly inadequate decision, and this was passed to the investigator's line manager to deal with. We got his response a few months later in July 2016. It was confusing and totally unhelpful in understanding the original decision and simply raised more questions than it answered.
It really did beggar belief that a statutory regulator could have produced such a sloppy and ill-reasoned response.
Needless to say, it essentially upheld the original decision. However, on the point of the unauthorised medicines being on sale, the manager decided there was now enough evidence and has issued 'formal advice' to the Superintendent Pharmacist at Nelsons, Suzanne Haar — this was to be kept on record and referred to whenever the site is next due for a routine inspection. We were not given a copy of this advice despite attempts to find out so we could better understand the legal position regarding these unlicensed products.
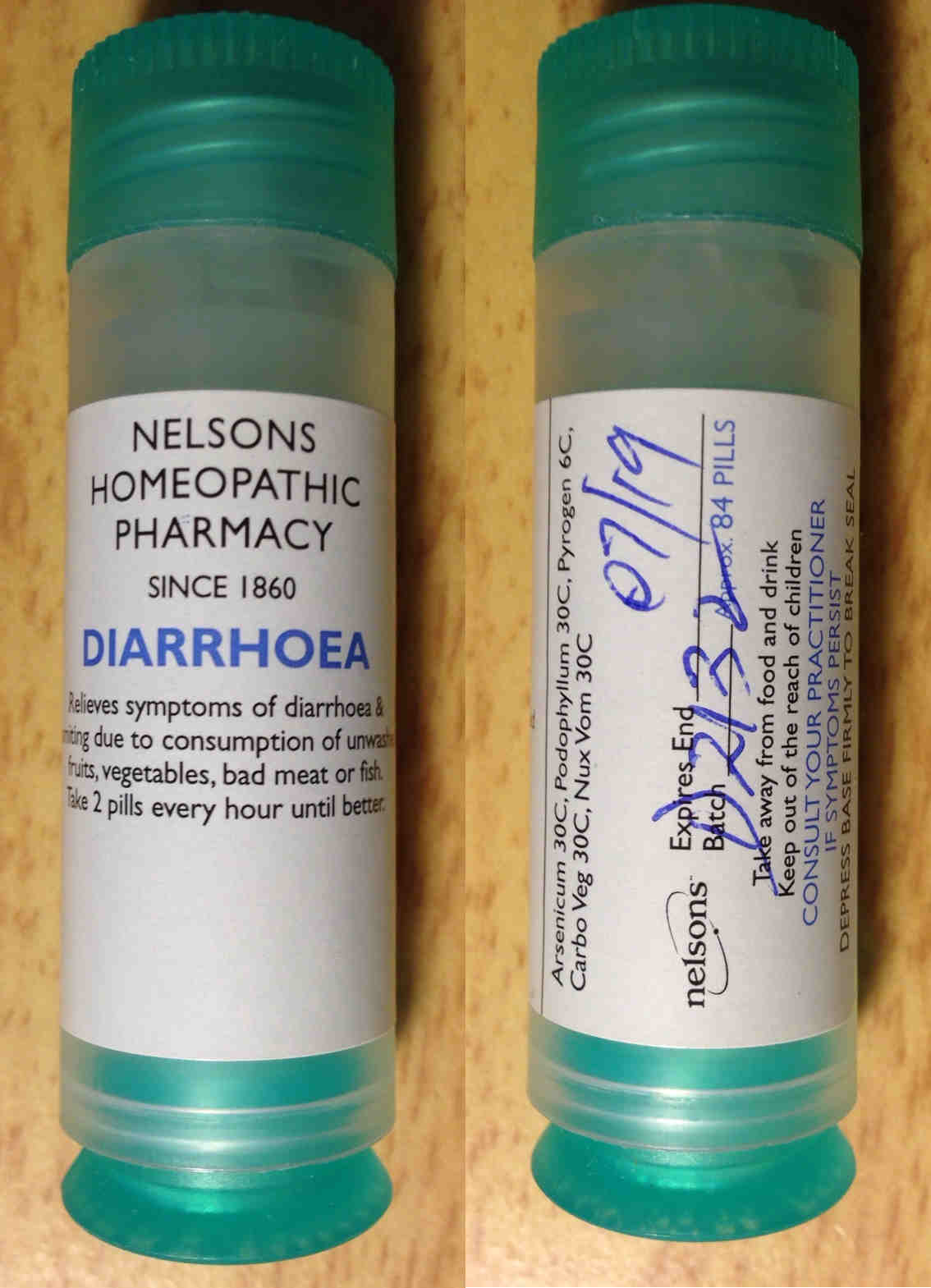 Complaint 2 Complaint 2
Because we were completely unsatisfied with this response, we paid Nelsons another on 28 July 2016. Guess what Maria was able to purchase? Exactly the same diarrhoea product she did the first time. We also noticed the same unauthorised products on sale as well as unauthorised tissue salts products. Perhaps the most galling thing was that the booklet Which remedy do I need? that was banned by the MHRA from Holland and Barrett was still there despite that being part of our complaint. It's not believable that Nelsons were not aware of our three MHRA complaints on this and the MHRA's decision.
It appears that despite our complaints to the MHRA and the GPhC and despite their investigations and the formal advice given to them, absolutely nothing had changed.
So, we responded to both the MHRA and the GPhC again with all we found and we were told that they were both considering this as new complaints against Nelsons and the pharmacist/pharmacists.
As we said in the last instalment, we kept it as a joint MHRA/GPhC complaint because we didn't want anything to fall through the cracks. We have copied correspondence to both regulators throughout: that seems to have been a wise move. We said:
We're sure you will be as concerned and as disappointed as we are that despite previous rulings that the POS [Point of Sale] material was not permitted and despite your previous action, this and other materials are still being used and that unauthorised medicines are being displayed and sold. It is difficult to understand why these obvious breaches have not been properly addressed by all concerned long before now and that we have had to submit a further complaint.
In summary, our concerns are that Nelsons are still:
- selling unauthorised medicinal products (with indications) in breach of the regulations.
- offering for sale on their premises and on their website unauthorised medicinal products (including herbal mother tinctures), many with indications.
- using point of sale materials that have indications for homeopathic medicinal products for which indications are not permitted.
- offering for sale Schuessler Salts products with indications in breach of your ruling on this after our previous complaint - I was unable to verify whether they were described as homeopathic or whether their dilution was given in homeopathic terms.
- offering for sale Dr Reckeweg 'tissue salts' products that are labelled with a 'homeopathic' dilution.
- advertising in their price list unauthorised medicinal products (including herbal mother tinctures), many of which have indications.
- advertising in their price list unauthorised kits of homeopathic products with indications.
…
We sincerely hope that our complaints will now be fully and thoroughly investigated and that any breaches of the regulations are finally dealt with.
Some of these points relate to the complaint to the MHRA so read the previous newsletter to find out what they are all about. The GPhC seemed a tad confused:
It is unclear at this stage whether these new concerns relate to the same matters that we have addressed in our recent investigation. Once we have conducted a full review of the documentation we will confirm whether we will be opening a new case. We will also consider if any aspects of your new concerns should be referred to the MHRA for further investigation.
It's difficult to understand what was unclear.
While we were waiting for the GPhC to deal with this, we received a reply from the MHRA telling us they had received confirmation from Nelsons that they had taken action to address points 1, 2, 3, 4, 5 and 7. We wanted to check to see what had changed, so we paid them another visit.
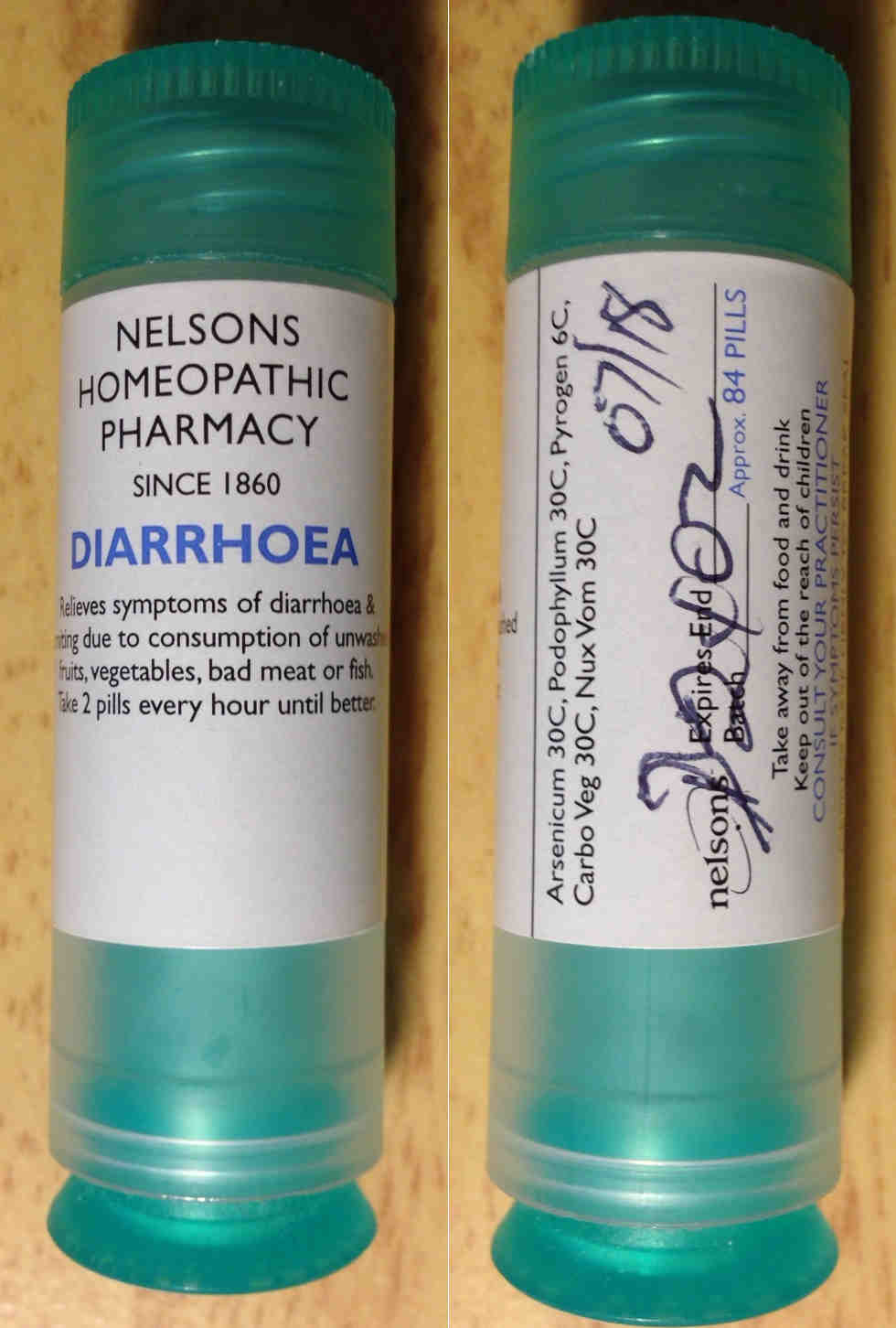 Complaint 3 Complaint 3
We told both the MHRA and the GPhC:
We are sure you will be as disappointed and frustrated as we are to discover that they still do not appear to have addressed all the issues.
Maria visited the shop this afternoon:
- She self-selected and purchased yet another tube of 'Diarrhoea Relief' homeopathic product.
- It is not known whether the member of staff who sold the Diarrhoea Relief product to Maria was a pharmacist or not (but we are certain it was not the Superintendent Pharmacist, Suzanne Haar), but she was asked no questions whatsoever about the product, who it was for or for what medical condition it was being purchased. The till receipt (see attached scan) gives the name 'Marlyn Hap'.
- The clear plastic boxes of these unauthorised medicinal products with lists of indications on the front were on two shelves in the same place in the shop as before and fully accessible to members of the public.
- There were two notices attached to the edge of the lower shelf. One said: 'Not for self selection' and the other said: 'Please ask a member of staff for help. Thank you' but it was not clear whether this referred to the products on the shelf to which it was attached or those on the shelf below. The upper shelf of unauthorised medicinal products had no such notices.
- The 'Which remedy do I need?' wire-bound point of sale promotional material for their registered and authorised homeopathic products was still attached to the rack of products.
- The Schuessler Tissue Salts products on the shelves gave the potency as a numeric value such as 6X on the front of the label. The display shelving unit (that housed all the above-mentioned products) was labelled 'Homeopathy' at the top.
This was our third complaint to both statutory regulators about the exact same issues. It has taken eighteen months thus far. We said:
As we said, it was disappointing that we had to submit a complaint the first time (and we would remind you that was on 28 May 2015). The various regulations surrounding the advertising, sale and supply of homeopathic products and unauthorised medicinal products are straightforward and it is the responsibility of the Pharmacists and Nelsons to ensure compliance. We are at a loss to understand why - after eighteen months and two previous complaints about essentially the same breaches - that these issues have not been fully and properly resolved. Your previous actions against Nelsons have clearly been wholly inadequate and it is disappointing that it has been necessary to submit yet another complaint.
We sincerely hope that the combined efforts of the MHRA and the GPhC will - this time - ensure that Nelsons Homeopathic Pharmacy finally comply with all the various regulations they must know they have to comply with.
We also said to the GPhC:
Now that the MHRA has concluded their second investigation and ruled that Nelsons Homeopathic Pharmacy have again breached various regulations concerning the advertising and supply of both unauthorised medicinal products and registered homeopathic products and herbal products, we would ask that you take these into account in your consideration of our previous complaint about the Registered Pharmacists at Nelsons Homeopathic Pharmacy and our further complaint detailed above.
It beggars belief that Nelsons thought that putting up a notice on the shelf saying 'Not for self selection' would be considered anywhere near adequate: the products were still available for self-selection.
Decision
The GPhC eventually referred the case to their Investigating Committee (IC) and a further eight months later in July 2017, we received their outcome. The allegations they considered were:
On 22 June 2017, the Investigating Committee considered the following allegations against Nelsons pharmacy, [Navreet Chaewla and Susanne Haar]:
- From 27 January 2014 to December 2016 the Pharmacy did not move all unlicensed medication as instructed from the front of the premises so that they were not available for self-selection in accordance with:
The action plan issued by the GPhC inspector on 27 January 2014; and
GPhC guidance, as set out in the letter dated 12 May 2016.
- Failed to ensure that the counter staff of Nelsons Pharmacy asked sufficient questions to determine that the treatment is suitable for the patient before the sale is completed
Haar is the Superintendent Pharmacist at Nelsons Homeopathic Pharmacy and, as such, takes responsibility for the way in which a company carries out its professional pharmaceutical activities. The decision they came to included:
In this case, the Investigating Committee decided to issue a warning to Susanne Haar and to give advice to Navreet Chaewla. This means that the Investigating Committee has concluded that the allegations need not be considered by the Fitness to Practise Committee. The Committee felt that it would be appropriate for the matter to be disposed of by the Investigating Committee.
In order to receive the warning, the committee had to adjourn and inform the registrant that that was what they were minded to do and gave the registrant the opportunity to make submissions about the proposed course of action or, if the registrant wished, to have the case heard by the Fitness to Practise Committee. In this case the registrant accepted a warning.
Please note that the letter of warning is a formal sanction that will stay on the registrant’s legal history with the GPhC for 5 years and on the public register for 2 years, and may be taken into consideration if any further allegations are made about the registrant.
We have also shared the outcome of the Investigating Committee with our local inspectors who may consider this as intelligence as part of any future inspection.
The warning placed on Haar's record is:
On 22 June 2017 the General Pharmaceutical Council’s Investigating Committee considered an allegation in relation to Mrs Susanne Skovgaard HAAR, registration number: 2039122 and determined to issue the registrant concerned with a warning in relation to the conduct alleged.
The warning has been issued to ensure public confidence in the profession and the regulatory process and to protect the public by reminding the registrant concerned to adhere to all legal and professional obligations in their practice in the future.
So despite all the issues we found, the multiple failings by Nelsons to abide by the regulations and to heed advice given to them by the regulators, it was not deemed to be a fitness to practice issue.
Although we received this last July, we were waiting for the MHRA to publish their final decision notice, which they did last week.
Three years, a 22-page initial complaint, two further complaints, two statutory regulators, multiple occasions where we had to chase both regulators and multiple occasions where we had to correct their misunderstandings and errors, but we finally got there.
Lessons
We did not do this to have anyone punished: we started this as an attempt to discover and understand precisely what pharmacies and pharmacists were and were not permitted to do in terms of selling homeopathic products and to test the statutory regulators' resolve to enforce those rules and regulations. The failures of Nelsons Homeopathic Pharmacy to implement the guidance provided by the regulators are what have caused this to have dragged on so long. But a complaint should never have been necessary in the first place: understanding the Human Medicines Regulations, the Medicines Act and all the other legislation surrounding the operation of pharmacies are part of the responsibilities of pharmacists. There can be no excuses. It should not have taken one complaint, never mind three, and it should not have taken nearly three years to resolve these very simple and straightforward issues.
What can we learn from this? What can homeopaths and homeopathic pharmacies learn from this? What, precisely, can pharmacies do in terms of selling homeopathic products, whether authorised, registered or neither?
This exercise has revealed a lot about all that, but that's for the next instalment.
26 February 2018
Share:    
Forward this e-mail to a friend
|

 So far, so good. But a major part of our complaint about
So far, so good. But a major part of our complaint about 
 Complaint 2
Complaint 2 Complaint 3
Complaint 3