News
News items will be published here, but also sign up to our Newsletters — see the form on the right.
You can receive updates from the News feed, either to your RSS reader or via email.
NHS Homeopathy: 20 years of decline
Data published today by NHS Digital shows that homeopathy prescriptions in England fell by nearly a quarter in 2016
Continuing its inexorable two-decade decline from its peak in 1996, the number of prescriptions for homeopathy products supplied in community pharmacies in England fell by 23% in 2016.
Data released today by NHS Digital show that homeopathy prescriptions fell from 8,894 in 2015 to 6,821 last year, a drop of 96% since its peak in 1996:

This drop is a welcome sign that the NHS continues to understand the need for robust evidence for treatments and that it does not exist for homeopathy. Although the mantra of choice is frequently repeated by supporters of homeopathy, providing a treatment for which there is no good evidence of specific patient benefit does not provide a choice to patients: they deserve better.
The House of Commons Science and Technology Select Committee in its Evidence Check on homeopathy concluded:
157. By providing homeopathy on the NHS and allowing MHRA licensing of products which subsequently appear on pharmacy shelves, the Government runs the risk of endorsing homeopathy as an efficacious system of medicine. To maintain patient trust, choice and safety, the Government should not endorse the use of placebo treatments, including homeopathy. Homeopathy should not be funded on the NHS and the MHRA should stop licensing homeopathic products.
In the most comprehensive review to date of the evidence for homeopathy, the Australian National Health and Medical Research Council concluded:
Based on the assessment of the evidence of effectiveness of homeopathy, NHMRC concludes that there are no health conditions for which there is reliable evidence that homeopathy is effective. Homeopathy should not be used to treat health conditions that are chronic, serious, or could become serious. People who choose homeopathy may put their health at risk if they reject or delay treatments for which there is good evidence for safety and effectiveness. People who are considering whether to use homeopathy should first get advice from a registered health practitioner. Those who use homeopathy should tell their health practitioner and should keep taking any prescribed treatments.
With such damning conclusions, patient choice cannot be heralded as sacrosanct. Consumers are perfectly free to buy their own homeopathy products, but it behoves the NHS to provide treatments that are based on robust scientific evidence.
Rising costs
The drop in the number of prescriptions is not matched by a corresponding fall in the total cost of those prescriptions: the number fell by 23% but the overall cost of these prescriptions only fell by 2%. How can this be?

For some reason, the average cost for each homeopathy item jumped by 28% to £13.55 compared to the previous year's £10.60. This is a substantial rise and may have been due to a jump in the cost of raw sugar… or maybe the suppliers wanted to try to compensate for the decreased sales volume? Looking at what happened in 2010, there could be a pattern.
Scotland
The next release of data for Scotland will be in June and will cover prescriptions from 01 April 2016 to 31 March 2017. There's no reason to suppose a similar decline won't be seen there too, particularly given the closure of the final seven in-patient beds at the Glasgow Homeopathic Hospital tomorrow: the legitimacy given to homeopathy by the NHS is slowly but surely being removed.
Popularity
Proponents of homeopathy frequently state that it's used by 10% of the population. They usually don't cite any evidence to substantiate that figure, but it could come from the statement made by Prof Kent Woods — the then Chief Executive of the MHRA — when giving evidence to the Select Committee in 2009 (Q182, Ev 65):
From the point of view of evidence, certainly from a regulatory perspective, it is very important evidence that something like ten per cent of the population have used a homeopathic remedy or have gone to a homeopath in the previous 12 months, and that I think is a starting point for deciding what is the public health significance of this phenomenon.
He didn't provide a source for that figure and it's always been contentious. Now, however, we have a more up-to-date figure from the European Social Survey. In their 2014 survey, they asked questions about the use of a number of CAM practices (acupressure, acupuncture, Chinese medicine, chiropractics, herbal treatment, homeopathy, hypnotherapy, massage therapy, osteopathy, physiotherapy, reflexology and spiritual healing) in the previous 12 months in 21 countries. For homeopathy, they found that only 1.3% of people had used it:

Ranking fifth, the UK compares very favourably with the other countries surveyed, and the usage is considerably less than previously claimed. It is also likely that even this is an over-estimate as the survey didn't provide any guidance to participants about what homeopathy was, so it's likely respondents may have included the use of all sorts of herbal or home remedies rather than just homeopathic products.
The same survey gives a picture of the usage of other practices in the UK:

However, for homeopathy on the NHS, it's difficult not to come to the conclusion that it's dying.
30 March 2017
The growing pains of osteopaths
The Advertising Standards Authority has today published new guidance on advertising claims made by osteopaths
Like all other advertisers, osteopaths have to comply with the ASA's CAP Code and the ASA/CAP publish specific guidance to help them — in addition to the more general guidance on health claims and their substantiation.
However, it seems that some osteopaths thought the CAP Code and guidance required some clarification.
Today, the ASA have published even more detailed guidance for those advertising osteopathy services.
This new document provides that clarity and further restricts the claims they can make about the use of osteopathy with pregnant women, children and babies, particularly non-musculoskeletal conditions such as colic and problems allegedly caused by 'birth trauma'.
A cursory glance at osteopaths' websites will find many such claims. See, for example, the results returned by this simple search for colic: osteopathy clinic site:.uk colic. This is a widespread problem.
Initiative
The new guidance is yet another joint initiative by the ASA and the osteopaths' statutory regulator, the General Osteopathic Council (GOsC) to reign in their registrants and they have written to all 4,800 UK of them informing them of the new guidance and the consequences of failing to comply:
The Osteopathic Practice Standards place a duty on all osteopaths to ensure their advertising complies with the CAP Code (Standard D14). Failing to comply with the Code and associated guidance or rulings could result in GOsC fitness to practise proceedings.
The GOsC and the osteopaths' trade body, the Institute of Osteopathy (IO) will be following this up with further communications and articles in various magazines and the GOsC will also be contacting the various osteopathy training organisations to make sure students are fully aware of their responsibilities. We hope that many students will ask questions of their tutors about why they are being taught something they are not allowed to advertise because of the lack of good evidence. Perhaps the GOsC should be asking themselves the same questions and revist their educational requirements.
Claims
There is a lot of work to be done by osteopaths.
For example, the Osteopathic Centre for Children — part of the Foundation for Paediatric Osteopathy whose tag line is "Paediatric osteopathy is the gold standard in holistic healthcare for children" — state:
Babies
An osteopathic check-up following the birth can help pin-point potential problems and helps to ease the dramatic transition from life inside the womb to the outside world. This initial adjustment involves many bodily systems such as breathing and digestion.
Stresses and strains from the labour or pregnancy can lead to unsettled behaviour and difficulties with feeding, winding, bowel movements and sleeping. Relieving any physical strains with gentle osteopathic treatment can be very helpful and relaxing. The care of the entire family unit is of the utmost concern for an osteopath.
Toddlers
This is a time when children learn to crawl, walk, run and communicate and are keen to explore their environment and to interact socially. It is desirable to monitor the progress of these early developmental milestones and to address the effects of any major physical mishaps or developmental lag to prevent problems developing in future.
In many ways these are mild claims; one osteopath's website more worryingly states:
OSTEOPATHY FOR BABIES AND CHILDREN
In the birthing process babies are subjected to enormous forces during their passage through the birth canal. Small amounts of movement exist in the infant skull to permit the baby’s head to adapt to these forces of labour. However when birth is difficult, unduly slow or fast, or complicated by the need for forceps ventouse or cesarean section delivery, the infant head may not fully recover from this distortion. If the baby is unable to resolve the stressors and strains naturally through breathing and suckling, these pressures with in the skull may lead to problems settling, feeding difficulties, disturbed sleep patterns and recurrent infections. Osteopathic treatment using a variety of gentle non invasive techniques may help improve the function of the musculoskeletal system and aid in the reduction of these symptoms.
During childhood, in addition to any earlier trauma or strains the body has to adapt with the growth and development of the bones and muscles. Postural changes and activity level changes with participation in sports can also produce aches and pains which may be eased with manual therapy. Restrictions in the musculoskeletal system can sometimes cause delay reaching developmental milestones for behaviour, speech and learning and may benefit from osteopathic assessment and treatment.
What parent would not be distressed by such stark warnings and sign their new-born up for immediate (and life-long?) treatment?
ASA guidance
Getting back the the ASA's new guidance on osteopathy, it states:
Following a review by CAP of the Bronfort et al Review in 2010, CAP accepts that osteopaths may claim to help a variety of medical conditions, including:
- generalised aches and pains,
- joint pains including hip and knee pain from osteoarthritis as an adjunct to core OA treatments and exercise
- arthritic pain,
- general, acute & chronic backache, back pain (not arising from injury or accident)
- uncomplicated mechanical neck pain (as opposed to neck pain following injury i.e. whiplash)
- headache arising from the neck (cervicogenic) / migraine prevention
- frozen shoulder/ shoulder and elbow pain/ tennis elbow (lateral epicondylitis) arising from associated musculoskeletal conditions of the back and neck, but not isolated occurrences
- circulatory problems,
- cramp,
- digestion problems,
- joint pains, lumbago,
- sciatica,
- muscle spasms,
- neuralgia,
- fibromyalgia,
- inability to relax,
- rheumatic pain,
- minor sports injuries and tensions.
The Bronfort review was commissioned by the General Chiropractic Council (GCC) in 2009 after I submitted 523 complaints to the GCC about claims being made by chiropractors on their websites — the GCC needed the review because they didn't have a clue about the evidence for the claims their registrants had been making. It was little more than a quick literature review carried out by chiropractors in the US.
Even though Bronfort et al. considered all manner of treatments including reflexology, massage and chiropractic manipulations and not just osteopathic techniques specifically, it did look at various paediatric conditions. In terms of the non-musculoskeletal conditions, Bronfort identified a number of systematic reviews and additional RCTs for these conditions. None was positive for any treatment.
However, it only gave results for the treatment of manual therapies for musculoskeletal conditions for adults and is silent on pregnant women, children and babies, so it's quite a leap to extrapolate to those patient groups from adults.
The National Council for Osteopathic Research (NCOR), funded by osteopaths and the GOsC, is currently undertaking a systematic review of manual therapies in the treatment of children and babies. It'll be interesting to see what they finally publish.
Meanwhile, it seems that the ASA believe that osteopaths are trained to treat pregnant women, children and babies and that they should therefore be allowed to make claims about these patient groups, despite being no good evidence that osteopathy is effective for these conditions. This simply raises the question as to what they are being taught if there is no good evidence it is effective for those groups in the first place.
The question of dose response is not addressed either nor the potential for harm — particularly the many claims that it is 'safe and gentle'.
However, that only applies to musculoskeletal conditions: the new guidance prevents them from directly or indirectly referring to conditions such as colic, growing pains, excessive crying and those allegedly caused by 'birth trauma'. As we have shown above, these are frequent claims.
Regulated
Because they are statutorily regulated, the ASA states osteopaths may:
…refer to conditions for which medical supervision should be sought if they hold convincing evidence of the efficacy of their treatments (Rules 12.1 and 12.2).
The caveat of holding the necessary standard of evidence is important, but it is not clear to us why statutory regulation should confer any special privileges: whilst the Osteopaths Act 1993 does provide for a means of regulating some aspects, it's primarily a means of protecting the title 'osteopath', ensuring registrants are appropriately insured and that there's a code of conduct and a complaints procedure. Notably, the Act does not prescribe any scope of practice, nor proscribe any treatments. This leaves the public open to being misled.
Principles
The new guidance sets out four principles:
Principle 1: Marketing claims
Claims made on osteopaths’ websites that serve the purpose of encouraging consumers to make a transactional decision (i.e. claims that directly or indirectly invite individuals to consider seeking osteopathic treatment for themselves or someone else must comply with the Advertising Code.
Principle 2: References to treating medical conditions
As healthcare practitioners regulated by statute, osteopaths may offer advice on, diagnosis of and treatment for conditions for which medical supervision should be sought. Those claims should be limited, however, to those for which the ASA or CAP has seen evidence for the efficacy of osteopathy for the particular condition claimed, or for which the advertiser holds suitable substantiation (references to conditions which the ASA or CAP accept osteopathy can help with should be understood on this basis, the ASA acknowledges that new evidence may emerge).2 The ASA retains the right to ask to review evidence for the purposes of resolving complaints should it consider the need to do so. Osteopaths should therefore ensure that they have access to substantiation before making such claims, including implied claims to treat a particular condition.
Principle 3: Substantiation for treatment claims
Where the efficacy of osteopathy for treating a particular condition has already been established, treatment claims that do not stray beyond the principles set out in the CAP Guidance will be considered compliant with the Code.
Principle 4: Osteopathy for general and specific patient populations
Osteopaths may make claims to treat general as well as specific patient populations, including pregnant women, children and babies provided they are qualified to do so. Osteopaths may not claim to treat conditions or symptoms presented as specific to these groups (e.g. colic, growing pains, morning sickness) unless the ASA or CAP has seen evidence for the efficacy of osteopathy for the particular condition claimed, or for which the advertiser holds suitable substantiation. Osteopaths may refer to the provision of general health advice to specific patient populations, providing they do not make implied and unsubstantiated treatment claims for conditions.
The principles don't say anything new, but the fourth one is important: osteopaths can claim to treat pregnant women, children and babies, but they can only do so in terms of the conditions listed above and it clearly states the conditions they should not claim to treat.
The guidance also gives some examples of claims that are likely and unlikely to be acceptable. The ASA, quite rightly, steer clear of saying outright what is and isn't acceptable: every claim has to be analysed individually in its proper context. We hope that osteopaths will take note of these carefully and not try to take advantage of any perceived equivocation.
Example claims which are unlikely to be acceptable:
- Osteopaths often work with crying, unsettled babies (Implies colic, which is not supported by evidence)
- Birth is a stressful process for babies
- Babies’ skulls are susceptible to strain or moulding, leading to asymmetrical or flattened head shapes. This usually resolves quickly but can sometimes be retained. Osteopathy can help
- If your baby suffers from excessive crying, sometimes known as colic, osteopathy might help
- Children often complain of growing pains in their muscles and joints; your osteopath can treat these pains
- Osteopathy can help your baby recover from the trauma of birth; I will gently massage your baby’s skull
- If your baby is having difficulty breastfeeding, osteopathy might be able to help
- Osteopathy can also play an important preventative role in the care of a baby, child or teenager and bring the body back to a state of balance in health
- In assessing a newborn baby, an osteopath checks for asymmetry or tension in the pelvis, spine and head, and ensures that a good breathing pattern has been established
- Cranial osteopathy releases stresses and strains in the skull and throughout the body
- Osteopaths can feel involuntary motion and mechanisms within the body
- Cranial osteopathy aims to reduce restrictions in movement
It might take osteopaths a while to clean up their websites.
All in the head
The weakest point of the new guidance is what it permits to be claimed about cranial osteopathy/craniosacral technique. It is a frequent treatment of choice by osteopaths for babies and children. Prof Ernst describes it:
Craniosacral therapy (CST), which, confusingly, is sometimes also called ‘cranial osteopathy’, was invented less than half a century ago by an osteopath. He thought that the spinal fluid is pulsating, the cranial bones are sufficiently movable to enable a therapist feel this pulse from the outside, and that it is possible to influence this process with very gentle manual manipulations which, in turn, would restore health in sick individuals. According to the inventor, the CST-practitioner uses his or her own hands to evaluate the craniosacral system by gently feeling various locations of the body to test for the ease of motion and rhythm of the fluid pulsing around the brain and spinal cord. Soft-touch techniques are then used to release restrictions in any tissues influencing the craniosacral system.
It's entirely fanciful, of course, but many osteopaths seem to believe it is effective for many childhood conditions and is seen by some as the 'bait and switch' used by some osteopaths to get new customers from a very early age.
But at least now they cannot claim that they can feel these movements nor that they can then manipulate the skull to alleviate anything.
Right direction
This guidance is a great step forward. Osteopaths now have absolute clarity about what they can and cannot claim for pregnant women, children and babies and we hope to see speedy changes to websites.
We also welcome the actions taken by the General Osteopathic Council to ensure their registrants stop misleading the public and we hope they will follow through when they are made aware of non-compliant websites; we also hope they will be pro-active in this and not simply wait around for others to submit complaints.
We now hope the ASA and the General Chiropractic Council will now do the same for chiropractors — it is long overdue and, arguably, a much larger problem. The list of allowed chiropractic claims will be shorter, of course, and the list of unacceptable claims longer. Much longer.
02 December 2016
The different faces of the Society of Homeopaths
The Society of Homeopaths seemed to be taking responsible action to curb the claims of their members. But what's been going on behind the scenes?
Little more than a month ago, the Society of Homeopaths (SoH) issued new advertising guidance to all their members. It was far from perfect and, we believe, strays some distance from the guidance laid down by the Advertising Standards Authority (ASA/CAP), but, being generous, at least it was a step in the right direction in curbing the worst excesses of its members' advertising claims.
 The ASA should now be checking the websites of SoH members and other homeopaths for compliance and taking appropriate action against those that fall short:
The ASA should now be checking the websites of SoH members and other homeopaths for compliance and taking appropriate action against those that fall short:
The ultimate sanction is referral by the ASA to the Trading Standards under the Consumer Protection from Unfair Trading Regulations 2008. Trading Standards is the legal backstop for the ASA. What this means is that where the threat or application of our sanctions have failed to achieve compliance, the matter may be formally referred to Trading Standards. Trading Standards will consider cases to determine if there are breaches of relevant legislation and take appropriate action in accordance with its own enforcement policy.
These are serious consequences for any homeopath and it may look like the SoH has faced up to its responsibilities and taken the responsible course of action in trying to help its members comply with the CAP Code and consumer protection legislation.
However, we now know that the SoH is not only supporting an anti-ASA campaign by some of its members, it is also seeking advice on the legality of the ASA's actions with a view to challenging them in the courts.
Legal challenge
Andy Lewis of The Quackometer has highlighted that the Society of Homeopaths (SoH) intend to fight the Advertising Standards Authority (ASA) over the advertising guidance they issued recently.
They're not doing this by providing high quality scientific evidence that substantiate claims for homeopathy made by their members, of course, but by trying to find a legal basis to undermine the ASA's legitimacy as the UK's independent advertising regulator.
They are also supporting a protest against the ASA, encouraging their members to complain to the Competition and Markets Authority and local Trading Standards. They seem to want them to:
…investigate allegations of illegal bias against homeopaths and homeopathy by the Advertising Standards Authority Ltd, in breach of Consumer Protection Regulations.
The 6 RSHoms claim that the ASA has no legal basis for what it is doing and is acting unreasonably in targeting homeopaths.
They are not clear on what consumer protection regulations they believe the ASA has breached, but it's odd that in their previous guidance to their members on complying with the ASA guidance, they stated:
The practical approach of the guidance, combines previous publications published by the Society of Homeopaths, CAP and ASA, and should allow further scope as to what is deemed acceptable, whilst remaining within current legislation, regulation and the Society’s Codes of Ethics. The document lays out a proposal, which has been cross-referenced against the following legislative and national statutory guidance sources. Most of the following are non-homeopathy specific and therefore applicable to all services across all industries:
This admission that the 'legislative and national statutory guidance' are not written specifically to cover errant homeopaths but are laws and regulations that are designed to protect consumers from misleading practices by any and all traders is interesting.
Their arguments against the ASA are well-worn and have been thoroughly refuted. They do try the old 'the ASA is only a limited company' meme and refer to them as the ASA Ltd: perhaps we should refer to the SoH by their own corporate title, The Society of Homeopaths Ltd?
Serious initiative
Anyway, in their guidance to their members, the SoH made several interesting statements with reference to the Professional Standards Authority (PSA) who legitimises them by placing them on their list of Accredited Registers. We submitted a Freedom of Information Act (FOIA) request to the PSA for details of all information held by the PSA relating to the SoH's guidance.
This provided several documents including an email from the SoH to the Director of Standards and Policy at the PSA, dated 07 December 2015:
Sorry to chase you but I wanted to update you on a serious initiative by ASA/CAP. See attached letter [download here].
In short, with no notice or consultation at all, they want to impose guidelines on us, send them out inside a month and then chase all our members to make sure they have complied within a month! You will not be surprised to hear that we have refused to co-operate but we have said that, if they pause the process, we are more than happy to meet with them and see if we can work out a way forward that ensures our members are compliant to reasonable guidelines. We need to be working with them in the long run, rather than working against them.
As far as I am aware, no other accredited register has been targeted. I am guessing they will be in time!
The belligerent nature of their language puts into context the SoH's latest pronouncement about taking legal action against the ASA and the assumption of being singled out by the ASA for special treatment speaks volumes. No doubt such a line would play nicely to their vexed members.
That the SoH refused to even cooperate with the ASA is extraordinary (even if they did recognise they will have to cooperate eventually) and we sincerely hope it raised more than a few eyebrows at the PSA.
The PSA themselves state:
Standard 8 requires registers to set standards of business practice, including advertising (and to comply with the Advertising Standards Authority’s requirements).
The PSA's 2015 decision to accredit the SoH gave the following instruction to the SoH:
The Society must replace the section of its Code of Ethics and Practice relating to Advertising and Media which states ‘Examples of Codes the Society may also take account of are the relevant clauses of The UK Code of Non-broadcast Advertising, Sales Promotion and Direct Marketing (CAP Code), and the current guidelines of the Society’ with ‘will take into account’. This action to be completed within three months.
The PSA's views of the necessity of complying with the ASA's rules could not be clearer, but here we have am Accredited Register not only wanting special, more 'reasonable' — presumably less stringent — privileges but actively seeking to take legal action against them.
Five years notice
But why this talk of 'no notice or consultation'? The ASA's remit was extended to cover traders' websites on 01 March 2011. The ASA issued clear guidance specifically for homeopaths in September 2011. The ASA's adjudication against the SoH themselves was published on 03 July 2013. There can surely be no excuse for the SoH not to be aware of their obligations under the CAP Code yet they and their members have already been given five years to comply. What have they not complied already? How much more time do they need?
It does seem to us that the SoH were looking to the PSA for some protection from those baddies at the ASA. However, other than an informal discussion between them on 12 January, no more appears to have been said. Hopefully the PSA will have reminded the SoH at that meeting of their responsibilities under the Accredited Register scheme.
But it seems the SoH were successful to some extent in delaying the ASA's action: their letter finally went out to homeopaths up and down the country in September this year and they were given a full five weeks in which to get their websites in order — five years eight months after the rules came into force for websites.
Hearsay
But returning to the SoH's guidance, we felt that what they said about testimonials was particularly interesting:
Testimonials
When referring to testimonials, it is wise to encourage people to seek independent medical advice and stay in close contact with their mainstream healthcare professionals. This supports an open dialogue, choice and informed, integrated care. The below was agreed by the Preliminary Investigation Panel, the Professional Standards Authority have also seen this statement, and appear happy with its use.
Declaring that the PSA 'appear happy' with their interpretation of the ASA's guidance on testimonials seemed rather odd and informal language to us: did the PSA fully agree with the statement or did they not? Did they even have a view on whether such a disclaimer would adequately protect them from the ASA?
We asked the PSA:
1. What communication has there been with the SoH on this?
The testimonials were discussed as part of last year’s reaccreditation process, in particular the annual review report, we sent all of the information except the information we consider exempt to you in November 2015. There have been no further discussions about the testimonials since this date.
2. What does the statement that you 'appear happy' with the use of these statements mean?
It is our understanding that this relates to the statement in the published panel decision in 2015. The decision reflects the fact that the panel was made aware of the above but made no specific comment for or against it.
3. Can you confirm that you told the SoH that the use of these statements by their registrants in their advertising was acceptable to you?
We have made no specific comment about this matter beyond what is said in the 2015 decision. This is because we consider this to be a matter for the ASA.
The 2015 decision referred to is no longer on the PSA's website, but in relation to the ASA and testimonials, it says:
The Society [of Homeopaths] also found guidelines relating to testimonials were restrictive and could prevent registrants from providing sufficient information for service users to make informed decisions. The Panel noted the Society has provided a disclaimer for registrants’ websites to make clear testimonials are not intended to make any false claims about homeopathy.
It's not at all clear to us how the SoH interpreted that as saying that the PSA 'appear happy' with the SoH's disclaimer, but now that the PSA have made it clear they believe it to be a matter not for them but for the ASA, we sincerely hope the SoH will update their guidance to clarify the PSA's position on this and update their guidance to bring it in line with the ASA's.
The SoH's advertising guidance does go on to say:
Please note: Any claims made within a testimonial are subject to the CAP Code. Therefore, if a testimonial makes an efficacy claim, the author’s permission should be sought to edit out the relevant parts.
Although this is an important part in the ASA's guidance on testimonials and endorsements, it is but one part of it. Overall, the SoH could have saved themselves a lot of bother by simply referring their members directly to the ASA's own comprehensive and clearly written guidance rather than regurgitating it and interpreting it, changing it in the process, inadvertently or otherwise.
Trade directories
As well as writing to thousands of homeopaths, we have been made aware that the ASA have also contacted at least one trade directory, informing them of the requirements to comply with the CAP Code.
Therapy Directory lists some 3,855 practitioners and premises in 34 different categories, including homeopaths. We were sent a copy of the text of an email from them, sent originally to a homeopath, explaining their decision to comply with the ASA's guidance:
I can appreciate how you and other Homeopaths currently feel and I hope that this email will help your understanding as to why we have made these changes.
Because this is now a legal requirement for advertising, as a marketing platform, we must abide by it. CAP were the ones that contacted us about the changes which is why we have actioned it.
I can see that you have already complied [sic] a detailed document raising your concerns, but please also have a look here: http://www.homeopathy-soh.org/images/ASASept2016/ASA-Guidance-6.pdf [the SoH's advertising guidance] as this may prove helpful for you when addressing this with the ASA / CAP. I would recommend contacting CAP as they are the ones that have implemented these changes and are therefore the ones to speak with.
Unfortunately, because we are a marketing platform and not therapists ourselves, as mentioned before we must adhere to these changes and won't be challenging this with either the ASA or CAP.
Please do keep us updated, I believe that you will receive a great amount of support from other Homeopaths that are currently experiencing the same.
It's good to see the ASA taking such a comprehensive approach to cleaning up this sector and Therapy Directory's responsible action to comply with the ASA's rules.
We hope all homeopaths, their trade bodies and other trade directories follow this responsible lead.
Crossroads
But to return to the SoH in particular: is there really a conflict between representing your members best interests and complying with the rules, regulations and laws they are supposed to?
That depends on what you believe is in your members' best interests. The SoH seemed to have taken a responsible approach to persuade its members to comply, but the other face they now show wants to challenge the ASA in the courts.
It'll be interesting to see how far they get, but it would be perverse if it was believed that continuing to defy the advertising regulator and face possible conviction under consumer protection regulations was in the best interests of any homeopath or of the SoH itself.
The Society of Homeopaths Ltd are at a crossroads: they need to grapple with the choice they face and decide whether they will go down the path of challenging the ASA or finally face up to their responsibilities as a supposed professional regulator, overseen by the Professional Standards Authority.
This will also be a challenge for the PSA: how they deal with a wayward SoH will be a test of their willingness and ability to properly oversee their Accredited Registers and properly protect the public.
22 November 2016
Diluting misleading claims - ASA update
The Advertising Standards Authority take strong action to bring advertising by homeopaths into compliance
In March 2011, our very first campaign was against the misleading advertising claims made by homeopaths on their websites. That was six years ago, and we gave the Advertising Standards Authority a huge headache: how to persuade homeopaths to abide by the same rules all advertisers have to abide by.
Without those advertising rules (in the form of the CAP Code), advertisers would have free rein to make whatever claims they wanted; it would be a wild-west for all sorts of cowboys and quacks and one where the poor consumer would suffer.
Homeopaths have featured in the ASA's list of adjudications and informally resolved cases over the years, but the ASA have recently preferred to let their Compliance Team deal with homeopathy advertisers because they had already extensively reviewed the evidence — notably through complaints about the Society of Homeopaths and homeopath Steve Scrutton, Media & Communications person at the Alliance of Registered Homeopaths — and had an established position on it. Read more about these adjudications in: Landmark decisions for homeopaths.
 A few advertisers have been somewhat recalcitrant, believing they could face down the ASA. Some even seemed to think the ASA could be ignored, but many have found that is not a sensible course of action and one that's not good for their business. However, many advertisers of complementary and alternative therapies appear in the ASA's list of Non-compliant online advertisers and others have been referred to Trading Standards (TS).
A few advertisers have been somewhat recalcitrant, believing they could face down the ASA. Some even seemed to think the ASA could be ignored, but many have found that is not a sensible course of action and one that's not good for their business. However, many advertisers of complementary and alternative therapies appear in the ASA's list of Non-compliant online advertisers and others have been referred to Trading Standards (TS).
Trading Standards have now successfully prosecuted 'Electronic Healing' (a provider of complementary and alternative therapies and devices) who had been referred to them by the ASA. TS have also had numerous websites taken down: they cannot be ignored.
We have no doubt that many homeopaths were just not aware of the advertising rules or the need to abide by them. Many will now comply because they understand the need for rules to protect consumers and are responsible traders who want to stay on the right side of the law. But not all.
Action
Today, the ASA announced that they have written to homeopaths across the UK to remind them of the rules that govern what they can and can’t say in their marketing materials, including on their websites. This included their previous Guidance for Advertisers of Homeopathic Services and FAQs about advertising regulation and the sanctions the ASA can impose.
It also builds on the advertising guidance published by the Society of Homeopaths (SoH) a few days ago, which had been reviewed by the ASA. You may remember that the SoH themselves fell foul of the ASA three years ago and an adjudication against numerous claims they made on Twitter and on their website found they had breached the CAP Code on multiple counts. Read more about the adjudication in: Landmark decisions for homeopaths.
While we generally welcome the guidance from the SoH, we believe this guidance is incorrect and misleading in places and goes beyond what we believe the CAP Code allows, but at least it's a step in the right direction. Whether they ensure their members now comply with the CAP Code remains to be seen.
With the ASA's letter to thousands of homeopaths, their published guidance on compliance and the SoH's letter to their members, there really is now no excuse whatsoever for homeopaths to continue to make misleading claims.
The ASA has given them a deadline of 03 November 2016 to change their websites to become compliant:
After the expiration of this period, we will carry out extensive monitoring spot checks. Homeopathy practices that have failed to comply will be contacted again. After this time, we will consider the application of appropriate sanctions.
Campaigning continues
This is the culmination of six years of campaigning for us — our initial campaign and the occasional prodding of the ASA and submitting the odd strategic complaint. Although there is much more still to do, we've done our bit to highlight the many issues with homeopathy advertising: it is now up to homeopaths to take the responsible action they know they must.
Perhaps the Society of Homeopaths could set a good example to its members by having a thorough review of its own website?
29 September 2016
NHS homeopathy in Scotland - on a shoogly peg
NHS Homeopathy in Scotland shows similar long-term decline to that in England
We've previously looked at how homeopathy in the English NHS has plummeted 95% in the last two decades. We now focus on what's been happening is Scotland.
The NHS in Scotland is separate from the NHS in England and they collate their prescription data separately, provided by the Information Services Division, a division of National Services Scotland, part of NHS Scotland. (Wales NHS and Health and Social Care in Northern Ireland are also separate and we may look at these in the future.)
Their Prescription Cost Analysis (PCA) data since 2001 (shortly after devolution in 1999) are available on one handy webpage, making them easier to find, with each spreadsheet covering the financial year from 01 April up to 31 March of the year given in the filename. The latest spreadsheet, PCA_2016.xlsx, therefore covers 01 April 2015 to 31 March 2016. Note that for consistency with the data for England, we have attributed the Scottish data for the year in the filename to the previous year in the charts below, ie the 2015/2016 data are attributed with 2015 to match the way the data for England have been attributed.
Tab 3 - BNF sub-section gives the data we need. As with the NHS Digital (the re-branded name for the HSCIC) data for England, the data relate to the NHS prescriptions dispensed in community pharmacies and by Dispensing Doctors in Scotland.
The prescription data are listed by BNF chapter, section and sub-section and the one for homeopathic (homoeopathic before 2013) preparations is 19.02.03. The data provided include the number of dispensed items, the Gross Ingredient Cost and the cost per item. Note that the costs given in the English data are called Net Ingredient Costs (NIC) but they are directly comparable: the Publication Report explains:
Comparability
The main measures of drug ingredient cost and volumes of items dispensed in the community are comparable across the UK countries. However it should be noted that the gross ingredient cost (GIC) within Scotland is equivalent to the net ingredient cost (NIC) in England, i.e. the reimbursement cost of drugs before any pharmacy discounts are applied.
Collating these data from each of the years gives the following:

A spreadsheet with these data can be downloaded here.
Anomaly
But why the small rise in prescriptions in 2012 and 2013? Was there a resurgence in public demand for homeopathy? Perhaps more GPs became convinced of its curative power? Maybe some new, compelling evidence for homeopathy hit the headlines?
Probably not. The answer is more prosaic: the closure of the pharmacy at the Glasgow Homeopathic Hospital (re-branded the Centre for Integrative Care (CIC) recently) in 2011 will have caused a small increase in the number of prescriptions dispensed in community pharmacies.
When Andy Lewis revealed the closure of the pharmacy in November 2011, he said:
The ratchet on NHS homeopathy continues to turn. It would appear that the homeopathic pharmacy at the Glasgow Homeopathy Hospital has been closed.
A note to local GPs is reminding them that they have no obligation to fill the hole left by this closure by prescribing homeopathy if patients ask for it.
The Glasgow Local Medical Committee notes that there has been a sudden surge in requests from patients to prescribe homeopathic sugar pills after they have been unable to get them at the hospital.
The prescription numbers before 2011 don't include those dispensed at the GHH/CIC, but those after 2011 are inflated by the ones GHH/CIC patients have had go to their local community pharmacy to have dispensed.
But note that this blip is more than wiped out by the falls in 2014 and 2015, leaving an overall 62% drop in the past ten years.
The drop isn't as great as the 95% in England, but it is still very significant. Referrals to the GHH/CIC have been stopped by various Scottish Health Boards, putting pressure on them — it would seem unlikely that the long-term downward trend will be reversed.
Pressure
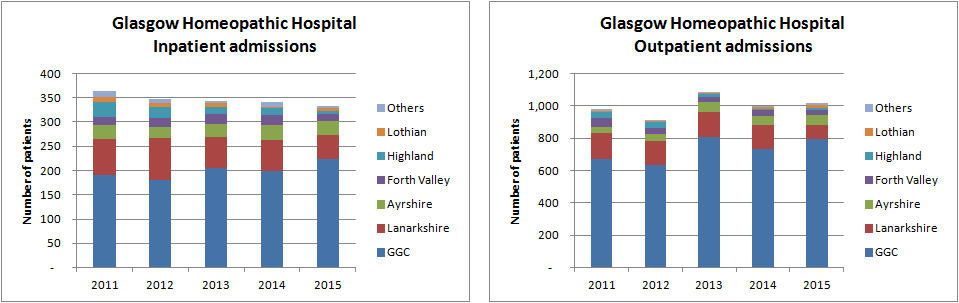
The number of patients at the GHH/CIC has been falling in recent years. Data from NHS Greater Glasgow and Clyde (GGC) — the health board that runs the GHH/CIC — show significant drops in the number of new inpatients from areas outside of GGCin recent years but that has been partially offset by an increase in numbers of referrals from within the GGC area. Overall, the number has still fallen 9% in the past four years to 332 in 2015 — about one new patient a day.
Similarly, falls in the number of new outpatients referred from areas outside GGC has been compensated by an increase in local referrals, leaving the number hovering around the 1,000 mark each year — in the order of three new patients a day. They also had 4,723 return appointments in 2015.
The hospital is currently facing the closure by GGC of its seven inpatient beds (which had previously been cut in 2010 from 15 beds Monday to Sunday to seven beds Monday afternoon to Friday morning), saving £190,000, putting it under further pressure.
In their proposal to close the seven inpatient beds, they said:
The proposal to close the CIC beds is based on the fact that:
- The Unit has reduced its inpatient service in recent years from a 15-bedded seven-day unit to only 7 beds, open 4 nights a week.
- The Centre has been very successful in developing an ambulatory model of care and all services are now available on that basis.
- Inpatient capacity is now underutilised delivering only 332 episodes of care each year. This will be further reduced by the continuing impact of decisions by other Boards to withdraw from the service, only 224 in patient episodes are provided for NHS GG and C residents.
- Inpatients account for only 5.2% of patient contacts for GGC residents. The majority of service delivery is already delivered in an outpatient setting.
Supporters of the GHH/CIC are campaigning to prevent its closure, including a Public Petition to the Scottish Parliament: PE01568: Funding, access and promotion of the NHS Centre for Integrative Care. The committee has been considering this for well over a year and seem to be making little progress; instead they seem keen to show their inability to understand scientific evidence.
If the closure is agreed by the GGC Board, this makes the GHH/CIC even less viable and it's not clear how long the hospital can last — its future is on a shoogly peg.
England and Scotland
As a reminder, the charts for England:

Combining these (from 2001 to 2015) give the following for England and Scotland together:

Homeopathy has been diluted to just 13% of its former self in the past 14 years.
Out of proportion
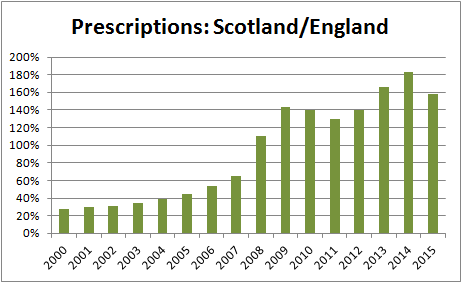 But looking at the data in more detail reveals something that looks odd: last year, there were 60% more prescriptions in total in Scotland than in England. The figures equate to 2.62 prescriptions per 1,000 population in Scotland but only 0.16 per 1,000 in England. But this has changed over the years: in 2001, it was the other way round with 2.7 times more prescriptions in England than in Scotland.
But looking at the data in more detail reveals something that looks odd: last year, there were 60% more prescriptions in total in Scotland than in England. The figures equate to 2.62 prescriptions per 1,000 population in Scotland but only 0.16 per 1,000 in England. But this has changed over the years: in 2001, it was the other way round with 2.7 times more prescriptions in England than in Scotland.
If it was simply down to population, you'd expect there to be more than ten times the number in England compared to Scotland.
Why is this not the case and why has it changed like this over the last 14 years?
One possible explanation might be to do with the number of NHS homeopathic 'hospitals' and the legitimacy they lend to homeopathy in general: there has only been the one in Scotland but there have been four in England in recent times: London, Bristol, Liverpool and Tunbridge Wells, plus a number of satellite clinics. Now, however, only the Royal London Hospital for Integrated Medicine remains, with the ones in both Liverpool and Bristol replaced by contracted private companies rather than being part of the NHS. The one in Tunbridge Wells closed in 2008. So, in its heyday around the turn of the century, Scotland was served by one but England was only served by four — proportionately far fewer considering the population.
So, with the only the London hospital now remaining — even though it no longer has a dedicated homeopathy service — the decline of prescriptions in England was perhaps inevitable, following the decline in the number of hospitals.
Good news
For those who do not believe public money should be spent on homeopathy, these figures will be welcome, but perhaps not so much for those in the homeopathy business as the false imprimatur given to homeopathy by the State plummets.
29 August 2016
Homeopathy on the NHS: at death's door
Homeopathy on the NHS falls for the eighteenth consecutive year
New figures released today show that homeopathy on the English NHS has fallen to a new low. The number of NHS prescriptions for homeopathy in England, fulfilled in community pharmacies, dropped by a further 13% in 2015 from the previous year and is 95% down from its peak nearly 20 years ago.
The data are compiled by the Health and Social Care Information Centre (HSCIC) — the official source of data for the NHS — and published as their Prescription Cost Analysis (PCA) data set.*
The new figures for 2015 show that there were just 8,894 prescriptions, down from 10,238 in 2014. The total cost of these prescriptions has dropped to £94,313, the first time it has been below £100,000.

The complete data for these charts are (all these figures can be verified from the original HSCIC data):
|
Year |
Prescription Items |
Net Ingredient Cost |
Cost/item |
|
1995 |
164,207 |
£816,798 |
£4.97 |
|
1996 |
172,013 |
£914,983 |
£5.32 |
|
1997 |
162,421 |
£937,311 |
£5.77 |
|
1998 |
157,063 |
£927,633 |
£5.91 |
|
1999 |
147,769 |
£888,274 |
£6.01 |
|
2000 |
134,164 |
£831,130 |
£6.19 |
|
2001 |
127,333 |
£807,125 |
£6.34 |
|
2002 |
117,989 |
£778,749 |
£6.60 |
|
2003 |
103,940 |
£714,938 |
£6.88 |
|
2004 |
94,501 |
£661,469 |
£7.00 |
|
2005 |
82,960 |
£593,316 |
£7.15 |
|
2006 |
62,679 |
£442,769 |
£7.06 |
|
2007 |
49,316 |
£321,418 |
£6.52 |
|
2008 |
26,337 |
£152,300 |
£5.78 |
|
2009 |
19,005 |
£100,486 |
£5.29 |
|
2010 |
16,359 |
£121,449 |
£7.42 |
|
2011 |
15,501 |
£130,601 |
£8.43 |
|
2012 |
15,262 |
£143,068 |
£9.37 |
|
2013 |
13,001 |
£137,298 |
£10.56 |
|
2014 |
10,238 |
£110,438 |
£10.79 |
| 2015 | 8,894 | £94,313 | £10.60 |
This follows on from other recent blows to NHS homeopathy: the closure of the homeopathy clinic at the South Bristol Community Hospital in Bristol, the reviews by both Liverpool CCG and Wirral CCG on ending the funding of homeopathy via the Liverpool Medical Homeopathy Service and other successfully completed reviews.
And the forthcoming Department of Health review of the blacklisting of homeopathy could mean CCGs are no longer able to prescribe it — not that many do now anyway.
Dilution by dilution, succussion by succussion, sugar pill by sugar pill, homeopathy is slowly but surely being removed from the NHS.
This will not be welcomed by homeopaths who businesses rely on the (undeserved and unearned) legitimacy that being provided on the NHS lends to homeopathy, but it's the inevitable result of the their own failure to provide robust evidence of its efficacy.
Switzerland legitimises homeopathy
In other news, however, it was announced by the international service of the Swiss Broadcasting Corporation, SwissInfo.ch, as:
Swiss to recognise homeopathy as legitimate medicine
…but it would have been more accurate to say:
Swiss to recognise homeopathy as if it were legitimate medicine
That's because:
…the interior ministry said it had come to the conclusion that it was “impossible to provide such proof for these disciplines in their entirety”.
So, it's not that the Swiss authorities had come across good evidence that homeopathy (and the other treatments covered) were, indeed, effective; more that they gave in and decided to reimburse them anyway, despite the lack of evidence.
The saga of homeopathy in Switzerland goes back many years (see That ‘neutral’ Swiss homeopathy report). Full and permanent inclusion in the Swiss state health reimbursement scheme from 2017 onwards was supposed to be contingent on being provided with evidence of "efficacy, cost-effectiveness and suitability" by 2015. Not surprisingly, homeopaths seem to have failed at that and the Swiss Government have completely ignored the findings of the comprehensive report by the Australian National Health and Medical Research Council that concluded:
Based on the assessment of the evidence of effectiveness of homeopathy, NHMRC concludes that there are no health conditions for which there is reliable evidence that homeopathy is effective. Homeopathy should not be used to treat health conditions that are chronic, serious, or could become serious. People who choose homeopathy may put their health at risk if they reject or delay treatments for which there is good evidence for safety and effectiveness. People who are considering whether to use homeopathy should first get advice from a registered health practitioner. Those who use homeopathy should tell their health practitioner and should keep taking any prescribed treatments.
It is therefore perverse that the Swiss Government should appear to bend over backwards to ignore the evidence and agree to pay homeopaths for dispensing their magic sugar pellets.
But it may not be as smooth a ride as the homeopaths might like. Reimbursement will only take place for treatments administered by certified medical doctors. Additionally, according to the official announcement in Komplementärmedizin soll anderen Fachrichtungen gleichgestellt werden, some criteria apply concerning tradition of usage and research, scientific evidence and medical experience and further education. Also, some treatments that are seen as critical are to be examined and potentially excluded from the reimbursement. It's not at all clear exactly what this will mean for the homeopaths, but this may not be the full endorsement from the Swiss Government they would like to believe.
Let's beware
This Sunday is the anniversary of the birth of the inventor of homeopathy, Samuel Hahnemann, heralding the start of World Homeopathy Awareness Week.
So as homeopathy on the NHS is in what must surely be its death throes, what better time to help others be aware of homeopathy?
Help spread the good news about homeopathy by sharing this newsletter and the following resources and further reading:
Skeptic successes in homeopathy by Jo Brodie
NHS Homeopathy Legal Challenge by the Good Thinking Society
NHS Homeopathy Spending by the Good Thinking Society
Should Homeopathic Remedies Be Blacklisted On The NHS? by the Good Thinking Society
Follow us on Twitter and re-Tweet our Tweets on homeopathy throughout World Homeopathy Awareness Week.
Notes
* For details of how to extract the data on homeopathy from the (very large) HSCIC data set, see: An idiot’s guide to understanding NHS homeopathy prescription data
07 April 2016
Latest news
- "Undisputable evidence of scientific misconduct" by homeopaths
- Yet another bad year for homeopathy
- Nelsons Homeopathic Pharmacy #3
- Nelsons Homeopathic Pharmacy #2
- The Society of Homeopaths: failing to make the case for homeopathy
- The end of homeopathy on the NHS in Bristol?
- NHS Homeopathy: 20 years of decline
- The different faces of the Society of Homeopaths
- The growing pains of osteopaths
- Diluting misleading claims - ASA update
Most read
- Finding deleted and changed webpages
- About The Nightingale Collaboration
- How to find out who owns a website
- Advertising Standards Authority
- Rubbing salts into the wounds of homeopathy
- How to submit a complaint to the ASA
- The decline of homeopathy on the NHS
- Landmark decisions for homeopaths
- NHS Lanarkshire to end referrals to Glasgow Homeopathic Hospital
- Making a complaint